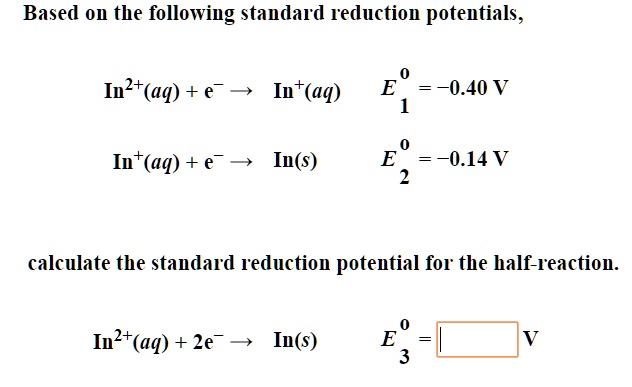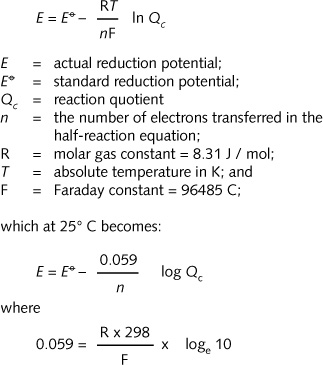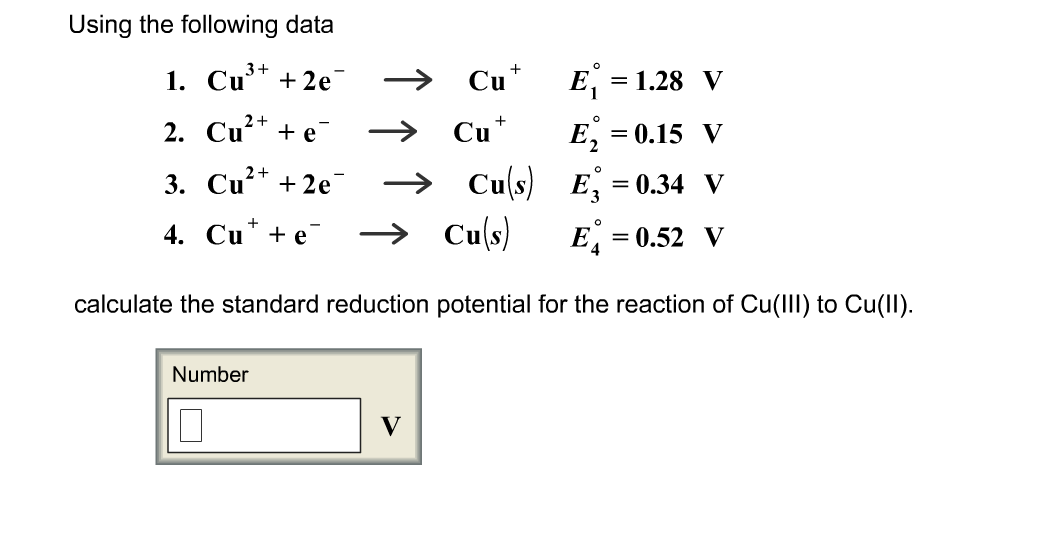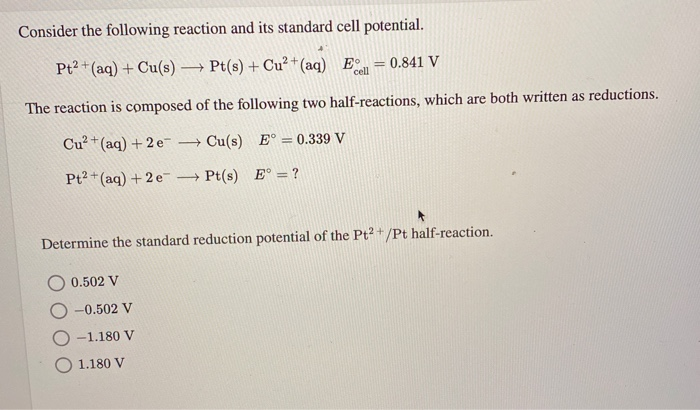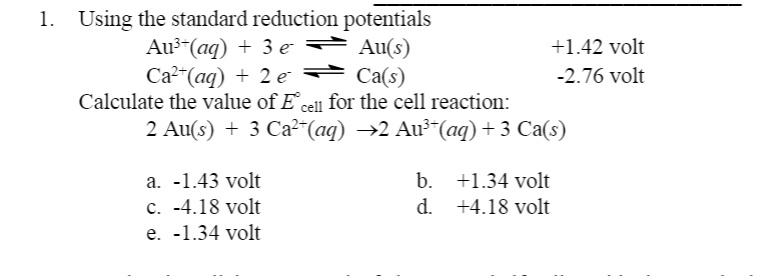
SOLVED: Using the standard reduction potentials Au'-(aq) 3 e Au(s) +1.42 volt Cal- -(aq) 1 2 e Ca(s) 2.76 volt Calculate the value of E cell for the cell reaction: Au(s) -
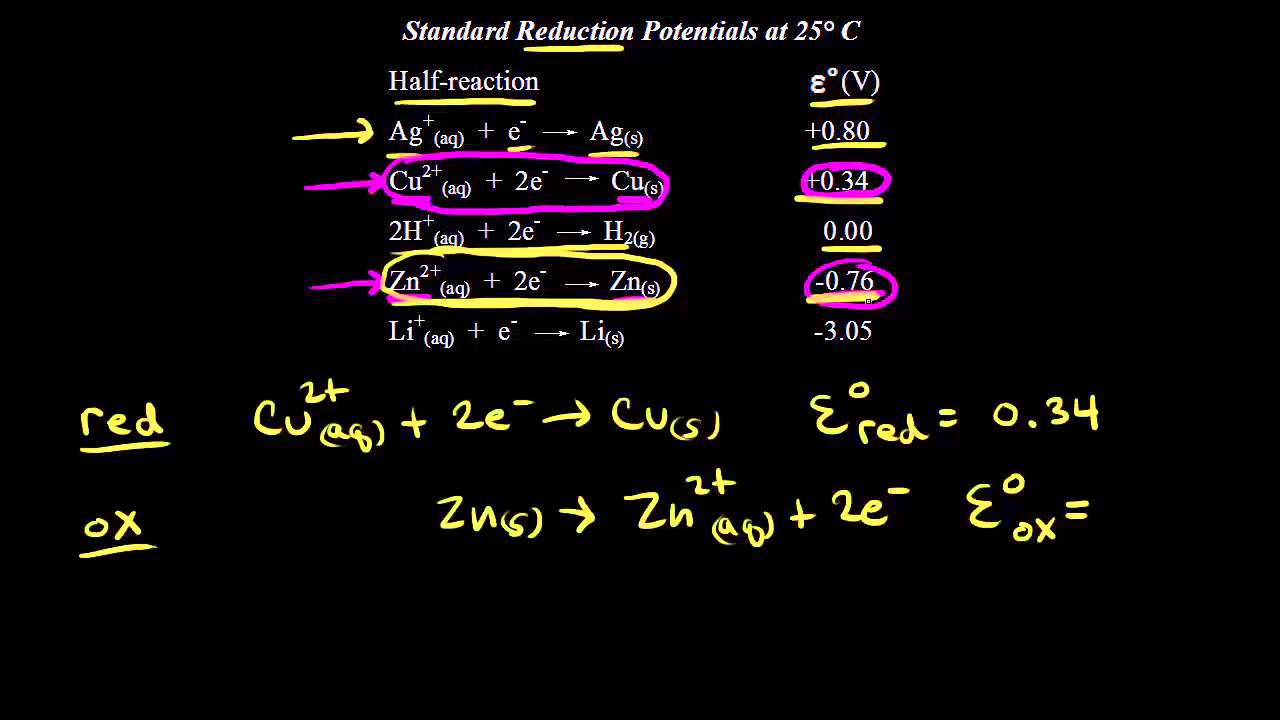
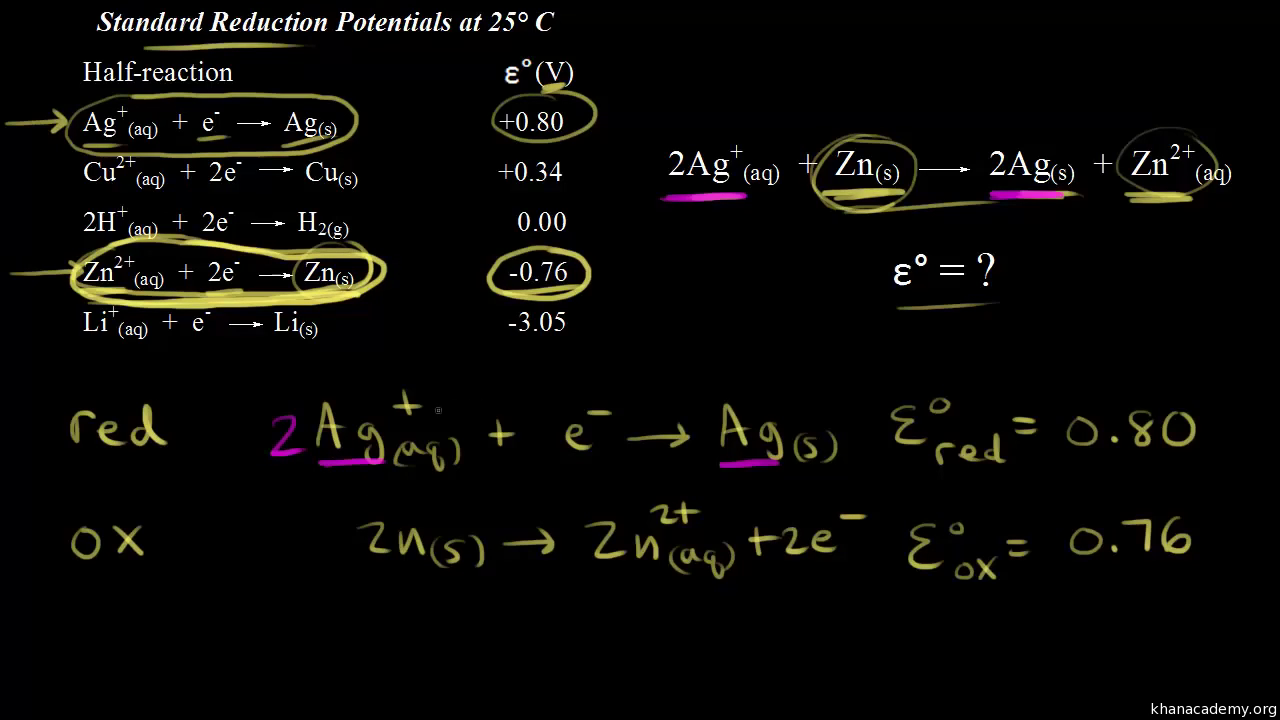
Standard reduction potentials | Redox reactions and electrochemistry | Chemistry | Khan Academy - YouTube

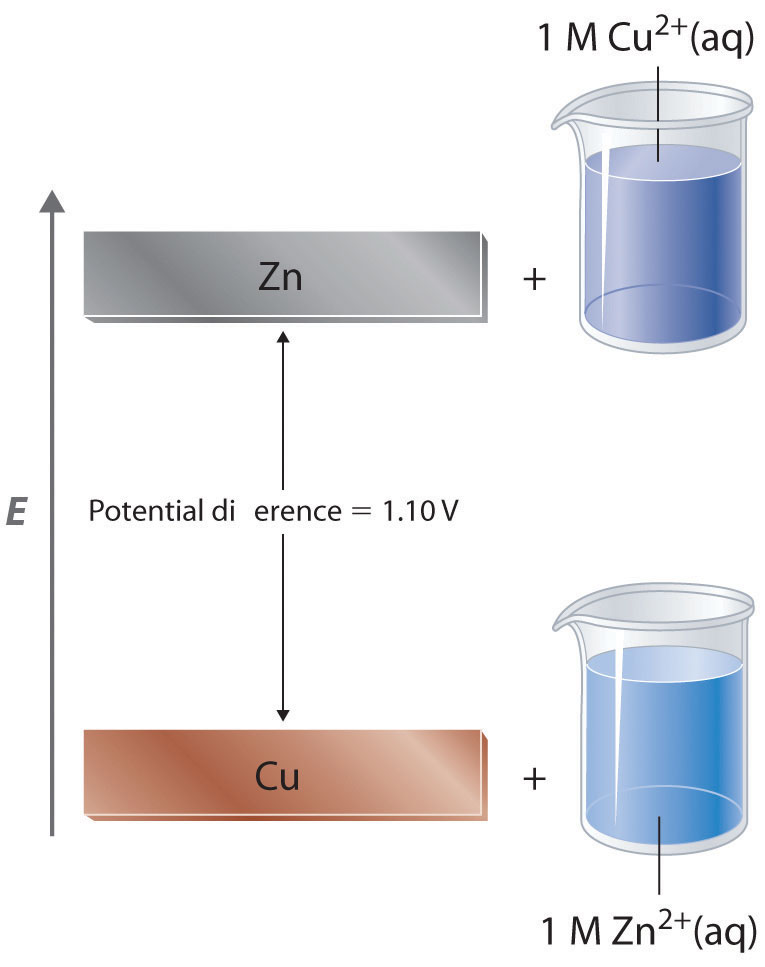
The standard reduction potential for `Cu^(2+)|Cu` is `+0.34V`. Calculate the reduction potential... - YouTube

The standard reduction potential for the half cell: NO3^-(aq.) + 2H^+(aq.) + e^ - → NO2(g) + H2O is 0.78 V. Calculate the reduction potential in 8M H^+ .

equilibrium - Calculate the cathode electrode potential in this redox reaction - Chemistry Stack Exchange

Question Video: Calculating a Cell Potential from Standard Electrode Potentials of Cadmium and Nickel | Nagwa

Question Video: Calculating the Standard Cell Potential for a Magnesium/Silver Galvanic Cell | Nagwa
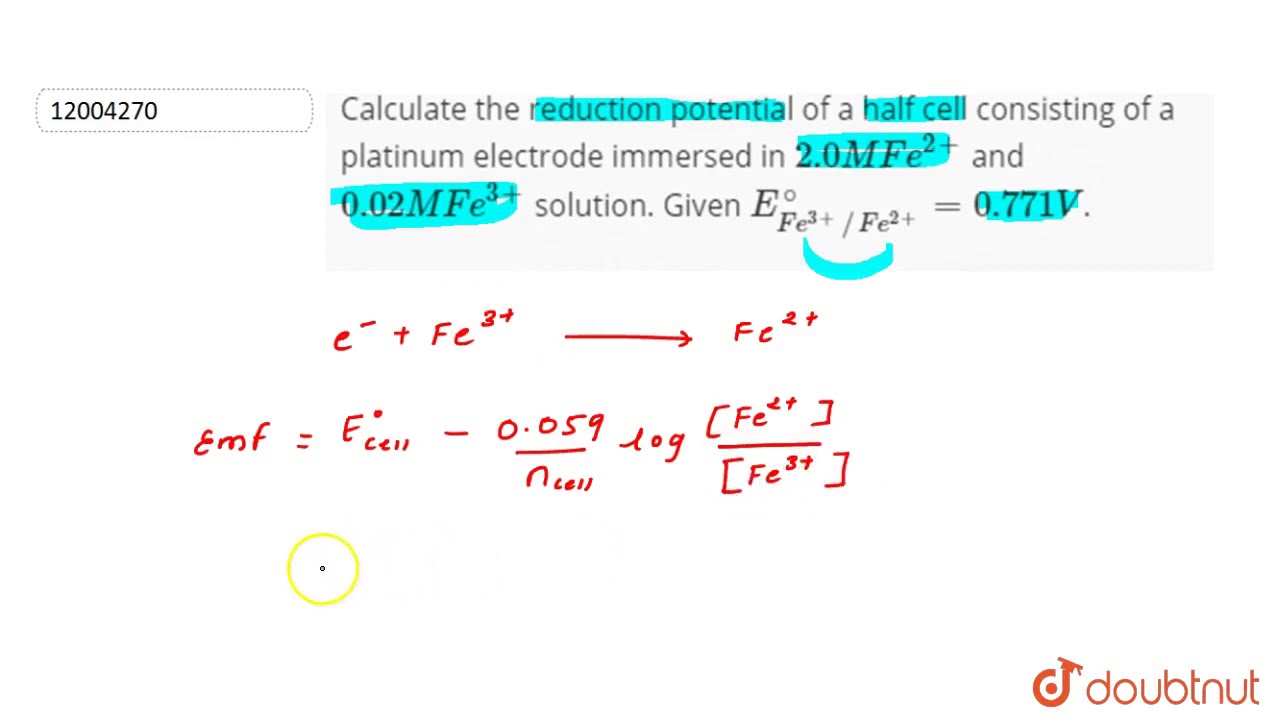
Calculate the reduction potential of a half cell consisting of a platinum electrode immersed in - YouTube

Calculate the reduction potential of a half cell consisting of a platinum electrode immersed in 2.0 MFe^2 and 0.02 M FE^3 solution. Given E^0Fe^3/Fe^+2 = 0.771 V .

Calculate the reduction potential of the following electrodes `:` `a.` `Pt,H_(2)(4 atm)|H_(2)SO_... - YouTube

OneClass: Given the following half-reactions and their respective standard reduction potentials calcu...